Haemolytic anaemia involves the destruction of red blood cells (haemolysis), resulting in a low haemoglobin concentration (anaemia).
Several inherited conditions cause the red blood cells to be more fragile and break down faster than normal, leading to chronic haemolytic anaemia. These include:
- Hereditary spherocytosis
- Hereditary elliptocytosis
- Thalassaemia
- Sickle cell anaemia
- G6PD deficiency
Several acquired conditions lead to the destruction of red blood cells:
- Autoimmune haemolytic anaemia
- Alloimmune haemolytic anaemia (e.g., transfusions reactions and haemolytic disease of newborn)
- Paroxysmal nocturnal haemoglobinuria
- Microangiopathic haemolytic anaemia
- Prosthetic valve-related haemolysis
Features
The features are a result of the destruction of red blood cells:
- Anaemia
- Splenomegaly (the spleen becomes filled with destroyed red blood cells)
- Jaundice (bilirubin is released during the destruction of red blood cells)
Investigations
The key investigation results are:
- Full blood count shows a normocytic anaemia
- Blood film shows schistocytes (fragments of red blood cells)
- Direct Coombs test is positive in autoimmune haemolytic anaemia (not in other types)
Hereditary Spherocytosis
Hereditary spherocytosis is the most common inherited haemolytic anaemia in northern Europeans. It is an autosomal dominant condition. It causes fragile, sphere-shaped red blood cells that easily break down when passing through the spleen.
It presents with anaemia, jaundice, gallstones and splenomegaly. A notable feature is aplastic crisis in the presence of the parvovirus. There is likely to be a positive family history.
Key findings are:
- Raised mean corpuscular haemoglobin concentration (MCHC) on a full blood count
- Raised reticulocyte count due to rapid turnover of red blood cells
- Spherocytes on a blood film
Treatment is with folate supplementation, blood transfusions when required and splenectomy. Gallbladder removal (cholecystectomy) may be required if gallstones are a problem.
Hereditary Elliptocytosis
Hereditary elliptocytosis is similar to hereditary spherocytosis except that the red blood cells are ellipse-shaped. It is also autosomal dominant. The presentation and management are the same as hereditary spherocytosis.
G6PD Deficiency
G6PD deficiency is caused by a defect in the gene coding for glucose-6-phosphate dehydrogenase (G6PD), an enzyme responsible for protecting the cells from oxidative damage. It is an X-linked recessive genetic condition (where males are more often affected and females are carriers). It is more common in Mediterranean, Asian and African patients.
The condition results in acute episodes of haemolytic anaemia triggered by infections, drugs or fava beans. Key medication triggers include ciprofloxacin, sulfonylureas (e.g., gliclazide) and sulfasalazine.
G6PD deficiency presents with jaundice (often in the neonatal period), gallstones, anaemia, splenomegaly and Heinz bodies on a blood film. Diagnosis can be made by doing a G6PD enzyme assay.
TOM TIP: The critical piece of knowledge for G6PD deficiency relates to triggers. In your exams, look out for a male patient that turns jaundiced and becomes anaemic after eating fava beans (broad beans), developing an infection or taking antimalarials. The underlying diagnosis might be G6PD deficiency.
Autoimmune Haemolytic Anaemia
Autoimmune haemolytic anaemia (AIHA) occurs when antibodies are created against the patient’s red blood cells. These antibodies lead to red blood cell destruction (haemolysis). There are two types, warm and cold, based on the temperature at which the auto-antibodies destroy red blood cells.
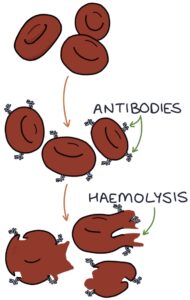
Warm autoimmune haemolytic anaemia is the more common type. Haemolysis occurs at normal or above-normal temperatures. It is usually idiopathic, meaning that it arises without a clear cause.
Cold-reactive autoimmune haemolytic anaemia is also called cold agglutinin disease. At lower temperatures (e.g., less than 10ºC), the antibodies attach to the red blood cells and cause them to clump together, called agglutination. The immune system is activated, and the red blood cells are destroyed. Cold AIHA can be secondary to lymphoma, leukaemia, systemic lupus erythematosus and infections (e.g., mycoplasma, EBV, CMV and HIV).
Management of autoimmune haemolytic anaemia involves:
- Blood transfusions
- Prednisolone
- Rituximab (a monoclonal antibody against B cells)
- Splenectomy
Alloimmune Haemolytic Anaemia
Alloimmune haemolytic anaemia occurs due to foreign red blood cells or foreign antibodies. The two scenarios where this happens are transfusion reactions and haemolytic disease of the newborn.
Haemolytic transfusion reactions occur when red blood cells are transfused into the patient. The immune system produces antibodies against antigens on the foreign red blood cells. An immune response leads to the destruction of those foreign red blood cells.
Haemolytic disease of the newborn occurs when maternal antibodies cross the placenta from the mother to the fetus. These maternal antibodies target antigens on the red blood cells of the fetus. These maternal antibodies destroy the neonate’s red blood cells. It occurs when the fetus is rhesus D positive (with rhesus D antigens on their red blood cells), and the mother is rhesus D negative (with no rhesus D antigens on her red blood cells). During a sensitisation event (e.g., antepartum haemorrhage), the mother can get exposed to the fetal red blood cells and start producing anti-D antibodies against the rhesus D antigen. In future, these antibodies can cross to the baby and cause haemolysis. Sensitisation is prevented in rhesus-negative women by using anti-D prophylaxis.
Paroxysmal Nocturnal Haemoglobinuria
Paroxysmal nocturnal haemoglobinuria is caused by a specific genetic mutation in the haematopoietic stem cells in the bone marrow. This mutation occurs during the patient’s lifetime (as opposed to being an inherited genetic condition). It results in a loss of the proteins on the surface of red blood cells that inhibit the complement cascade, allowing activation of the complement cascade on red blood cells and their destruction.
The characteristic presenting symptom is red urine in the morning, which contains haemoglobin and haemosiderin. Other presenting features are anaemia, thrombosis (e.g., DVT, PE and hepatic vein thrombosis) and smooth muscle dystonia (e.g., oesophageal spasm and erectile dysfunction).
Management is with eculizumab or bone marrow transplantation. Eculizumab is a monoclonal antibody that targets complement component 5 (C5). Bone marrow transplantation can be curative.
Microangiopathic Haemolytic Anaemia
Microangiopathic haemolytic anaemia (MAHA) involves the destruction of red blood cells as they travel through the circulation. This is most often caused by abnormal activation of the clotting system, with blood clots (thrombi) partially obstructing the small blood vessels, referred to as thrombotic microangiopathy. These obstructions churn the red blood cells, causing haemolysis (rupture). Picture a mesh inside the small blood vessels shredding the red blood cells.
Microangiopathic haemolytic anaemia is usually secondary to an underlying condition, such as:
- Haemolytic uraemic syndrome (HUS)
- Disseminated intravascular coagulation (DIC)
- Thrombotic thrombocytopenic purpura (TTP)
- Systemic lupus erythematosus (SLE)
- Cancer
Schistocytes are a key finding on the blood film in patients with microangiopathic haemolytic anaemia.
Prosthetic Valve Haemolysis
Haemolytic anaemia is a key complication of prosthetic heart valves. It occurs in both bioprosthetic and metallic valve replacement, although it varies depending on the type. It is caused by turbulence flow around the valve and the shearing of the red blood cells. The valve churns up the cells, and they break down.
Management involves:
- Monitoring
- Oral iron and folic acid supplementation
- Blood transfusions if severe
- Revision surgery may be required in severe cases
Last updated August 2023
Now, head over to members.zerotofinals.com and test your knowledge of this content. Testing yourself helps identify what you missed and strengthens your understanding and retention.

