Infective endocarditis refers to infection of the endocardium (the inner surface) of the heart.
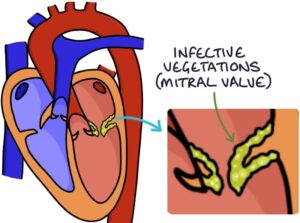
It causes infective vegetations in the heart, which are clumps of fibrin, platelets and bacteria.
Most commonly, it affects the heart valves, such as the mitral valve, aortic valve or tricuspid valve.
It can be acute or subacute, depending on how rapidly and acutely the symptoms present and the causative organism.
Risk Factors
The risk factors for infective endocarditis are:
- Intravenous drug use
- Structural heart pathology (see below)
- Chronic kidney disease (particularly on dialysis)
- Immunocompromised (e.g., cancer, HIV or immunosuppressive medications)
- History of infective endocarditis
Structural pathology can increase the risk of endocarditis:
- Valvular heart disease
- Congenital heart disease
- Hypertrophic cardiomyopathy
- Prosthetic heart valves
- Implantable cardiac devices (e.g., pacemakers)
Causes
The most common cause is Staphylococcus aureus.
Other causes include:
- Streptococcus (notably the viridans group of streptococci)
- Enterococcus (e.g., Enterococcus faecalis)
- Rarer causes include Pseudomonas, HACEK organisms and fungi
Presentation
The presenting symptoms are non-specific for an infection:
- Fever
- Fatigue
- Night sweats
- Muscle aches
- Anorexia (loss of appetite)
The key examination findings are:
- New or “changing” heart murmur
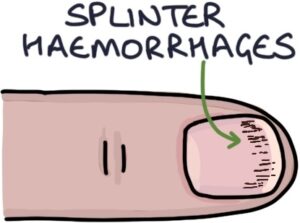
- Splinter haemorrhages (thin red-brown lines along the fingernails)
- Petechiae (small non-blanching red/brown spots) on the trunk, limbs, oral mucosa or conjunctiva
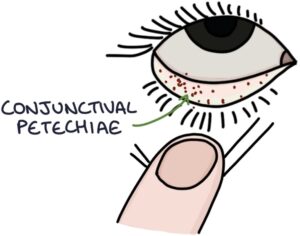
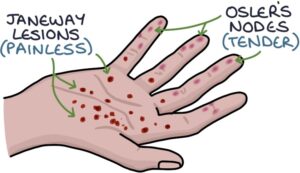
- Janeway lesions (painless red flat macules on the palms of the hands and soles of the feet)
- Osler’s nodes (tender red/purple nodules on the pads of the fingers and toes)
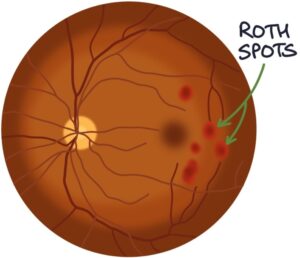
- Roth spots (haemorrhages on the retina seen during fundoscopy)
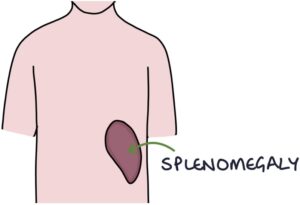
- Splenomegaly (in longstanding disease)
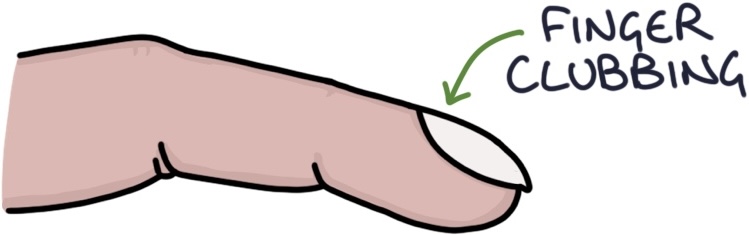
- Finger clubbing (in longstanding disease)
Investigations
Blood cultures are essential before starting antibiotics. Three blood culture samples are recommended, usually separated by 30 minutes and taken from different sites.
Echocardiography is the usual imaging investigation. Transoesophageal echocardiography (TOE) is more sensitive and specific than transthoracic echocardiography. Vegetations (an abnormal mass or collection) may be seen on the valves.
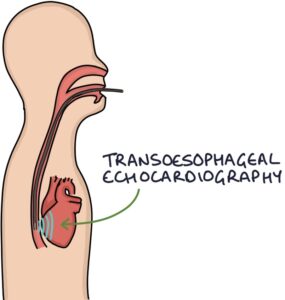 Special imaging investigations may be used in patients with prosthetic heart valves, where it can be more challenging to determine whether an infection is present in the prosthesis:
Special imaging investigations may be used in patients with prosthetic heart valves, where it can be more challenging to determine whether an infection is present in the prosthesis:
- 18F-FDG PET/CT
- SPECT-CT
Modified Duke Criteria
The Modified Duke criteria can be used to diagnose infective endocarditis. A diagnosis requires either:
- One major plus three minor criteria
- Five minor criteria
Major criteria are:
- Persistently positive blood cultures (typical bacteria on multiple cultures)
- Specific imaging findings (e.g., a vegetation seen on the echocardiogram)
Minor criteria are:
- Predisposition (e.g., IV drug use or heart valve pathology)
- Fever above 38°C
- Vascular phenomena (e.g., splenic infarction, intracranial haemorrhage and Janeway lesions)
- Immunological phenomena (e.g., Osler’s nodes, Roth spots and glomerulonephritis)
- Microbiological phenomena (e.g., positive cultures not qualifying as a major criterion)
Management
Patients require admission and are managed by the relevant specialist team (e.g., the infective endocarditis or infectious diseases team).
Intravenous broad-spectrum antibiotics (e.g., amoxicillin and optional gentamicin) are the mainstay of treatment. The choice of antibiotic may be more specific once the causative organism is identified on cultures. Antibiotics are typically continued for at least:
- 4 weeks for with native heart valves
- 6 weeks for patients with prosthetic heart valves
Surgery may be required for:
- Heart failure relating to valve pathology
- Large vegetations or abscesses
- Infections not responding to antibiotics
Infective endocarditis has a high mortality rate. Key complications include:
- Heart valve damage, causing regurgitation
- Heart failure
- Infective and non-infective emboli (causing abscesses, strokes and splenic infarction)
- Glomerulonephritis, causing renal impairment
Prophylaxis
There are NICE guidelines (last updated in 2016) on the prophylaxis of infective endocarditis. Antibiotics are not routinely recommended for dental and non-dental procedures as prophylaxis of infective endocarditis. However, it is still considered on a case-by-case basis in those at particularly high risk.
Patients at higher risk are advised to take good care of their oral health to reduce the risk of infective endocarditis.
Last updated March 2023
Now, head over to members.zerotofinals.com and test your knowledge of this content. Testing yourself helps identify what you missed and strengthens your understanding and retention.