Atrial fibrillation (AF) is a condition where the electrical activity in the atria of the heart becomes disorganised, leading to fibrillation (random muscle twitching) of the atria and an irregularly irregular pulse.
The overall effects of atrial fibrillation are:
- Irregularly irregular ventricular contractions
- Tachycardia (fast heart rate)
- Heart failure due to impaired filling of the ventricles during diastole
- Increased risk of stroke
Pathophysiology
Normally, the sinoatrial node produces organised electrical activity that coordinates the contraction of the atria. Atrial fibrillation occurs when this electrical activity is disorganised, causing the contraction of the atria to become uncoordinated, rapid and irregular. This chaotic electrical activity overrides the regular, organised activity from the sinoatrial node. It passes through to the ventricles, resulting in irregularly irregular ventricular contraction.
Uncoordinated atrial activity means the blood can stagnate in the atria, forming a blood clot (thrombus). A thrombus formed in the left atrium may travel to the brain and block a cerebral artery, causing an ischaemic stroke. The risk of stroke is about 5 times higher than usual in patients with atrial fibrillation (depending on individual factors).
Common Causes
The most common causes of atrial fibrillation can be remembered with the “SMITH” mnemonic:
- S – Sepsis
- M – Mitral valve pathology (stenosis or regurgitation)
- I – Ischaemic heart disease
- T – Thyrotoxicosis
- H – Hypertension
Alcohol and caffeine are lifestyle causes worth remembering.
Presentation
Patients are often asymptomatic, and atrial fibrillation is an incidental finding. It may be diagnosed after a stroke.
Patients may present with:
- Palpitations
- Shortness of breath
- Dizziness or syncope (loss of consciousness)
- Symptoms of associated conditions (e.g., stroke, sepsis or thyrotoxicosis)
The key examination finding is an irregularly irregular pulse. There are two differential diagnoses for an irregularly irregular pulse:
- Atrial fibrillation
- Ventricular ectopics
Ventricular ectopics disappear when the heart rate gets above a certain threshold. Therefore, a regular heart rate during exercise suggests a diagnosis of ventricular ectopics.
Investigations
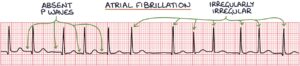
An ECG is required in all patients with an irregularly irregular pulse. The ECG findings in atrial fibrillation are:
- Absent P waves
- Narrow QRS complex tachycardia
- Irregularly irregular ventricular rhythm
An echocardiogram may be required to investigate further in cases of:
- Valvular heart disease
- Heart failure
- Planned cardioversion
Paroxysmal Atrial Fibrillation
Paroxysmal atrial fibrillation refers to episodes of atrial fibrillation that reoccur and spontaneously resolve back to sinus rhythm. These episodes can last between 30 seconds and 48 hours.
Patients with a normal ECG and suspected paroxysmal atrial fibrillation can have further investigations with:
- 24-hour ambulatory ECG (Holter monitor)
- Cardiac event recorder lasting 1-2 weeks
Valvular Atrial Fibrillation
Valvular atrial fibrillation is AF with significant mitral stenosis or a mechanical heart valve. The assumption is that the valvular pathology has led to atrial fibrillation. Atrial fibrillation without valve pathology or with other valve pathologies, such as mitral regurgitation or aortic stenosis, is classed as non-valvular AF.
The NICE guidelines (2021) do not reference valvular atrial fibrillation. They recommend patients with valvular heart disease are referred to a cardiologist for further assessment and management.
Principles of Management
Management here is based on the NICE guidelines from 2021. Follow local and national guidelines when treating patients.
There are two principles to treating atrial fibrillation:
- Rate or rhythm control
- Anticoagulation to prevent strokes
TOM TIP: The following details on rate and rhythm control get quite detailed and complex. Most patients will end up on a beta blocker for rate control, often bisoprolol, plus a DOAC for anticoagulation. If you remember one thing about the treatment of atrial fibrillation, remember this combination.
Rate Control
The function of the atria is to pump blood into the ventricles. In patients with atrial fibrillation, the atrial contractions are not coordinated, so the ventricles must fill by suction and gravity, which is considerably less efficient. The higher the heart rate, the less time is available for the ventricles to fill with blood, reducing the cardiac output. Rate control aims to get the heart rate below 100 and extend the time during diastole for the ventricles to fill with blood.
NICE guidelines (2021) suggest all patients with AF should have rate control as first-line, except with:
- A reversible cause for their AF
- New onset atrial fibrillation (within the last 48 hours)
- Heart failure caused by atrial fibrillation
- Symptoms despite being effectively rate controlled
Options for rate control:
- Beta blocker first-line (e.g., atenolol or bisoprolol)
- Calcium-channel blocker (e.g., diltiazem or verapamil) (not preferable in heart failure)
- Digoxin (only in sedentary people with persistent atrial fibrillation, requires monitoring and has a risk of toxicity)
Rhythm Control
Rhythm control may be offered to patients with:
- A reversible cause for their AF
- New onset atrial fibrillation (within the last 48 hours)
- Heart failure caused by atrial fibrillation
- Symptoms despite being effectively rate controlled
Rhythm control aims to return the patient to normal sinus rhythm. This can be achieved through:
- Cardioversion
- Long-term rhythm control using medications
For cardioversion, there is a choice between:
- Immediate cardioversion
- Delayed cardioversion
Immediate cardioversion is used if the atrial fibrillation is either:
- Present for less than 48 hours
- Causing life-threatening haemodynamic instability
There are two options for immediate cardioversion:
- Pharmacological cardioversion
- Electrical cardioversion
For pharmacological cardioversion, the options are:
- Flecainide
- Amiodarone (the drug of choice in patients with structural heart disease)
Electrical cardioversion aims to shock the heart back into sinus rhythm. It involves using a cardiac defibrillator machine to deliver controlled shocks. This is usually done with sedation or general anaesthesia.
Delayed cardioversion is used if the atrial fibrillation has been present for more than 48 hours and they are stable. Electrical cardioversion is recommended. Transoesophageal echocardiography‑guided cardioversion is an option where available. Amiodarone may be considered before and after electrical cardioversion to prevent AF from recurring.
The patient should be anticoagulated for at least 3 weeks before delayed cardioversion. During the 48 hours before cardioversion, they may have developed a blood clot in the atria, and reverting them to sinus rhythm carries a high risk of mobilising that clot, causing a stroke. They are rate controlled whilst waiting for cardioversion.
Long-term rhythm control is with:
- Beta blockers first-line
- Dronedarone second-line for maintaining normal rhythm where patients have had successful cardioversion
- Amiodarone is useful in patients with heart failure or left ventricular dysfunction
Management of Paroxysmal Atrial Fibrillation
Patients with paroxysmal atrial fibrillation may be appropriate for a “pill-in-the-pocket” approach. They take a pill to terminate their atrial fibrillation only when they feel the symptoms starting. To be suitable for a pill-in-the-pocket approach, they must have infrequent episodes without structural heart disease. They also need to be able to identify the signs of atrial fibrillation and understand when to take the treatment.
Flecainide is the usual treatment for a pill-in-the-pocket approach. There is a risk of flecainide converting the atrial fibrillation into atrial flutter, with 1:1 AV conduction to the ventricles, causing a very fast ventricular rate.
Patients with paroxysmal atrial fibrillation should still be anticoagulated based on their CHA2DS2-VASc score, similar to permanent atrial fibrillation.
Ablation
Where drug treatment for rate or rhythm control is not adequate or tolerated, there are two options for ablation:
- Left atrial ablation
- Atrioventricular node ablation and a permanent pacemaker
Left atrial ablation is performed in a catheter laboratory, often called a “cath lab”. It involves a general anaesthetic or sedation. A catheter is inserted into a femoral vein and fed through the venous system under x-ray guidance to the heart. The catheter punctures through the septum into the left atrium. Once in the left atrium, it is placed against different areas to test the electrical signals. The operator attempts to identify the location of any abnormal electrical pathways. Once identified, radiofrequency ablation (heat) is applied to burn the abnormal area of electrical activity. This leaves scar tissue that does not conduct electrical activity. The aim is to remove the source of the arrhythmia and restore normal sinus rhythm.
Atrioventricular node ablation involves destroying the connection between the atria and ventricles (the atrioventricular node). It is a catheter procedure. After the procedure, the irregular electrical activity in the atria cannot pass through to the ventricles. A permanent pacemaker is required to control ventricular contraction (the pacemaker is inserted before the ablation procedure). Anticoagulation is still needed to prevent strokes.
Anticoagulation
Uncontrolled and unorganised activity in the atria leads to blood stagnating in the left atrium, particularly in the left atrial appendage. Eventually, this stagnated blood leads to a thrombus (clot). This thrombus then mobilises (becomes an embolus) and travels from the left atrium to the left ventricle, into the aorta and up in the carotid arteries to the brain. It can then lodge in a cerebral artery and cause an ischaemic stroke.
Anticoagulation treatment reduces coagulation (thrombus formation) by interfering with the clotting cascade. Without anticoagulation, patients with atrial fibrillation have around a 5% risk of stroke each year, depending on individual factors. With anticoagulation, patients with atrial fibrillation have around a 1-2% risk of stroke each year, depending on individual factors. Anticoagulation reduces the risk of stroke by about 2/3.
Anticoagulation treatment carries around a 2.5-8% risk of serious bleeding each year, depending on individual factors.
The NICE guidelines (2021) recommend for anticoagulation:
- Direct-acting oral anticoagulants (DOACs) first-line
- Warfarin second-line, if DOACs are contraindicated
TOM TIP: Every patient with a head injury whilst taking anticoagulation should have a CT head to assess for an intracranial bleed, according to the NICE guidelines on head injuries (updated 2019). Being asked to review a patient after a fall is very common, and it helps to remember that a head injury plus anticoagulation automatically qualifies them for a CT scan. Inform patients starting on anticoagulation that they will need medical attention (A&E) in the event of a head injury for this reason.
Direct-Acting Oral Anticoagulants
Direct-acting oral anticoagulants (DOACs) are oral anticoagulants that do not require INR monitoring, unlike warfarin. They are suitable for most patients, including patients with cancer. They have a 6-14 hour half-life.
Apixaban, edoxaban and rivaroxaban are direct factor Xa inhibitors. Dabigatran is a direct thrombin inhibitor.
Apixaban and dabigatran are taken twice daily, and edoxaban and rivaroxaban are taken once daily.
Some of the DOACs have agents available to reverse the effects in uncontrolled or life-threatening bleeding:
- Andexanet alfa (apixaban and rivaroxaban)
- Idarucizumab (a monoclonal antibody against dabigatran)
DOACs have several advantages compared with warfarin:
- No monitoring is required
- No issues with time in therapeutic range (provided they have good adherence)
- No major interaction problems
- Equal or slightly better than warfarin at preventing strokes in atrial fibrillation
- Equal or slightly lower risk of bleeding than warfarin
The most common indications for DOACs are:
- Stroke prevention in patients with atrial fibrillation
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE)
- Prophylaxis of venous thromboembolism (DVTs and PEs) after a hip or knee replacement
Warfarin
Warfarin is a vitamin K antagonist. Vitamin K is essential for the functioning of several clotting factors. Warfarin blocks vitamin K. It prolongs the prothrombin time, which is the time it takes for blood to clot.
The INR (international normalised ratio) is used to assess how anticoagulated the patient is by warfarin. The INR calculates the patient’s prothrombin time compared with the prothrombin time of an average healthy adult. An INR of 1 indicates a normal prothrombin time. An INR of 2 means the patient has a prothrombin time twice that of an average healthy adult (it takes them twice as long to form a blood clot).
Warfarin requires close monitoring of the INR and frequent dose adjustments to keep the INR in range. It is given once daily, usually around 6 pm when in the hospital, so the INR is available before deciding the dose. The target INR for AF is 2 – 3.
Time in therapeutic range (TTR) refers to the percentage of time that the INR is in the target range. When the INR is too low, the patient is at increased risk of a stroke. When the INR is too high, the patient is at increased risk of bleeding.
The metabolism of warfarin involves the cytochrome P450 system in the liver. Therefore, the INR will be affected by other drugs that influence the activity of the P450 system, including many antibiotics. When starting new medications, the INR needs close monitoring, and the warfarin dose needs to be adjusted accordingly.
INR is also affected by many foods, particularly those that contain vitamin K, such as leafy green vegetables, and those that affect the P450 system, such as cranberry juice and alcohol. This means it is important to monitor the INR more closely when the patient changes their diet.
Vitamin K can reverse the effects of warfarin in the event of a very high INR or significant bleeding. Warfarin has a half-life of 1-3 days.
CHA2DS2-VASc
CHA2DS2-VASc is a tool for assessing whether a patient with atrial fibrillation should start anticoagulation. It is a list of risk factors that increase the likelihood of a stroke. The higher the score, the higher the risk of developing a stroke or TIA.
CHA2DS2-VASc is a mnemonic for the factors that score a point:
- C – Congestive heart failure
- H – Hypertension
- A2 – Age above 75 (scores 2)
- D – Diabetes
- S2 – Stroke or TIA previously (scores 2)
- V – Vascular disease
- A – Age 65 – 74
- S – Sex (female)
NICE (2021) recommends, based on the CHA2DS2-VASc score:
- 0 – no anticoagulation
- 1 – consider anticoagulation in men (women automatically score 1)
- 2 or more – offer anticoagulation
Aspirin alone is not used for stroke prevention in atrial fibrillation (using aspirin was an option years ago).
Bleeding Risk
The NICE guidelines recommend using the ORBIT score for assessing the risk of major bleeding in patients with atrial fibrillation taking anticoagulation. The easiest way to calculate the ORBIT score is using an online calculator. The “ORBIT” mnemonic can be used to remember the 5 factors:
- O – Older age (age 75 or above)
- R – Renal impairment (GFR less than 60)
- B – Bleeding previously (history of gastrointestinal or intracranial bleeding)
- I – Iron (low haemoglobin or haematocrit)
- T – Taking antiplatelet medication
For most patients with atrial fibrillation, the risk of stroke with no anticoagulation will outweigh the risk of bleeding on anticoagulation.
Left Atrial Appendage Occlusion
Left atrial appendage occlusion is an option for patients with contraindications to anticoagulation and a high stroke risk. The left atrial appendage is a small pouch in the wall of the left atrium. It is the most common site for a thrombus to form. Left atrial appendage occlusion involves inserting a catheter into the femoral vein, feeding that through the venous system to the right atrium and puncturing the septum between the atria to access the left atrium. Then, a plug is placed in the left atrial appendage, preventing blood from entering that area.
Last updated March 2023
Now, head over to members.zerotofinals.com and test your knowledge of this content. Testing yourself helps identify what you missed and strengthens your understanding and retention.

