Haemoglobin is the molecule responsible for transporting oxygen around the body. It is found in red blood cells. It picks up oxygen in the lungs, transports it through the blood and releases it in the tissues where it can be used. In the fetus, haemoglobin is slightly different, because it needs to pick up oxygen in the placenta, stealing it from the mothers haemoglobin.
Haemoglobin is formed of four protein subunits. These four subunits are made of two pairs of subunits. Fetal haemoglobin (HbF) has two alpha and two gamma subunits. Adult haemoglobin (HbA) has two alpha and two beta subunits.
Differences with Adult Haemoglobin
The structure gives fetal haemoglobin a greater affinity to oxygen than adult haemoglobin. Oxygen binds to fetal haemoglobin more easily and is more reluctant to let go. This is important, as fetal haemoglobin needs to “steal” oxygen away from the mother’s haemoglobin when nearby in the placenta. If the fetal and maternal haemoglobin had the same affinity for oxygen, there would be no incentive for the oxygen to switch from the maternal blood to fetal blood.
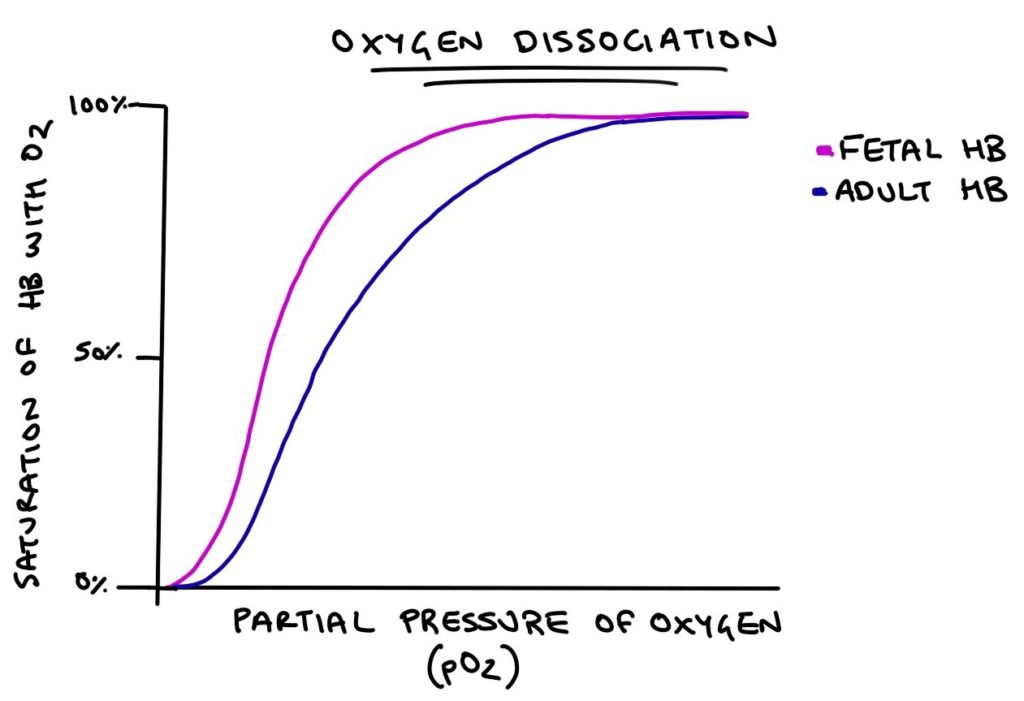
The affinity of fetal and adult haemoglobin with oxygen can be illustrated with the oxygen dissociation curve. This is an exam favourite. Along the x-axis is the partial pressure of oxygen, which is how much oxygen is crammed into a space. The higher the partial pressure, the more oxygen is in the area. On the y-axis is the percentage of the haemoglobin molecule that is bound to oxygen. This is how “full” the haemoglobin molecule is.
As the partial pressure of oxygen goes up, more oxygen will be bound to haemoglobin. Adult haemoglobin requires a higher partial pressure of oxygen for the molecule to fill with oxygen compared with fetal haemoglobin.
At birth
From 32 to 36 weeks gestation, production of HbF decreases. At the same time HbA is produced in greater quantities. Over time there is a gradual transition from HbF to HbA. At birth, around half the haemoglobin produced is HbF and half is HbA. By 6 months of age, very little fetal haemoglobin is produced. Eventually, red blood cells contain entirely HbA.

Fetal Haemoglobin in Sickle Cell Disease
In sickle cell disease, a genetic abnormality coding for the beta subunit is responsible for causing the sickle shape of the red blood cells. Fetal haemoglobin does not lead to sickling of red blood cells because there is no beta subunit in the structure.
Hydroxycarbamide can be used to increase the production of fetal haemoglobin (HbF) in patients with sickle cell anaemia. This has a protective effect against sickle cell crises and acute chest syndrome.
Last updated January 2020
