Thalassaemia is caused by a genetic defect in the protein chains that make up haemoglobin. Normal haemoglobin consists of two alpha-globin and two beta-globin chains.
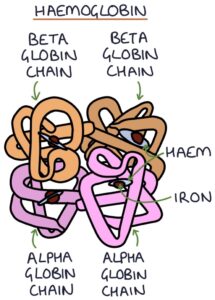
Defects in alpha-globin chains lead to alpha thalassaemia. Defects in the beta-globin chains lead to beta thalassaemia. Both conditions are autosomal recessive. The overall effect is varying degrees of anaemia, depending on the type and mutation.
In patients with thalassaemia, the red blood cells are more fragile and break down easily, causing haemolytic anaemia. The spleen acts as a sieve, filtering the blood and removing older cells. The spleen collects all the destroyed red blood cells, resulting in splenomegaly.
Features
The severity of features depends on the type. Universal features include:
- Microcytic anaemia (low mean corpuscular volume)
- Fatigue
- Pallor
- Jaundice
- Gallstones
- Splenomegaly
- Poor growth and development
Investigations
Microcytic anaemia (low mean cell volume) is a typical finding on a full blood count. Raised ferritin suggests iron overload.
Haemoglobin electrophoresis is used to diagnose globin abnormalities. DNA testing can be used to look for the genetic abnormality.
All pregnant women in the UK are offered a screening test for thalassaemia at booking.
Iron Overload
Iron overload may occur in thalassaemia due to:
- Increased iron absorption in the gastrointestinal tract
- Blood transfusions
Iron overload in thalassaemia can cause symptoms and complications of:
- Liver cirrhosis
- Hypogonadism
- Hypothyroidism
- Heart failure
- Diabetes
- Osteoporosis
Serum ferritin levels are monitored. Management involves limiting transfusions and iron chelation.
Alpha-Thalassaemia
Defects in the alpha-globin chains cause alpha-thalassaemia. The genes that code for alpha-globin are found on chromosome 16. The severity of symptoms varies depending on the type and number of genetic defects, ranging from entirely asymptomatic as a carrier, to moderate anaemia (haemoglobin H disease), to intrauterine death due to severe fetal anaemia (alpha thalassemia major).
Management involves:
- Monitoring
- Blood transfusions
- Splenectomy may be performed
- Bone marrow transplant can be curative
Beta-Thalassaemia
Defects in beta-globin chains cause beta-thalassaemia. The gene coding for this protein is on chromosome 11. The gene defects can either consist of abnormal copies that retain some function or deletion genes with no function in the beta-globin. Based on this, beta-thalassaemia can be split into three types:
- Thalassaemia minor
- Thalassaemia intermedia
- Thalassaemia major
Thalassaemia Minor
Patients with beta thalassaemia minor (also called thalassaemia trait) are carriers of an abnormally functioning beta-globin gene. They have one abnormal and one normal gene.
Thalassaemia minor causes mild microcytic anaemia and usually only requires monitoring.
Thalassaemia Intermedia
Patients with beta thalassaemia intermedia have two abnormal copies of the beta-globin gene. This can be either:
- Two defective genes
- One defective gene and one deletion gene
Thalassaemia intermedia causes more significant microcytic anaemia. Patients require monitoring and may need occasional blood transfusions. They may require iron chelation to prevent iron overload.
Thalassaemia Major
Patients with beta thalassaemia major are homozygous for the deletion genes. They have no functioning beta-globin genes. This is the most severe form and usually presents with severe anaemia and failure to thrive in early childhood.
The bone marrow is under so much strain to produce extra red blood cells to compensate for the chronic anaemia that it expands enough to increase the risk of fractures and change the patient’s appearance. Abnormal features relating to bone changes include:
- Frontal bossing (prominent forehead)
- Enlarged maxilla (prominent cheekbones)
- Depressed nasal bridge (flat nose)
- Protruding upper teeth
Management involves regular transfusions, iron chelation and splenectomy. A bone marrow transplant can be curative.
Last updated August 2023
